Understanding Electron Relative Mass | A GCSE Perspective, Relativistic Mass Overview & Important Highlights
The electron is one of the fundamental particles that constitute the universe. Even though it is very small, it is very important in the realm of chemistry, physics, and technology. One important aspect concerning an electron is its relative mass because it always brings up questions such as, “What is the relative mass of an electron in GCSE?” or “The relative mass of an electron is 1/1840 or 1/2000?” In this blog, we will look into the relativity of an electron and its relativistic mass and also seek to answer why these values are so important in science.
What is the Relative Mass of an Electron in GCSE?
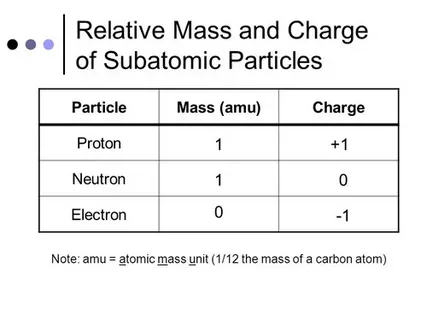
The concept of ‘relative mass’ in GCSE physics and chemistry refers to a massless particle that is used to compare the electron’s weight against a proton or neutron’s weight. Given the fact that electrons are significantly lighter than protons and neutrons, this value is given in such a way to be a fraction.
In GCSE relative mass of an electron is approximately equal to 1/1840 which would mean that the electron weighs approximately 1840 times lesser than a proton or neutron. For example:
The mass of a proton is approximately 1 atomic mass unit (amu).
The mass of an electron is approximately 1 over 1840 amu.
This value is utilized in the GCSE curriculum for the reason that it suffices in aiding pupils grasp the concept of the atom’s constituents without undertaking intricate calculations.
What is the Relativistic Mass of an Electron?
From the perspective of Einstein’s theory of relativity, the relativistic mass of an electron is a consideration. It concerns the additional mass given to an object as it draws nearer to the light speed. For an electron, this is important when it is in a state of very high motion, for example within particle accelerators.
Here’s how it works:
1. Rest Mass:
The rest mass of an electron is its mass when it is not in motion, which is about 9.109 x 10^-31 kilograms.
2. Relativistic Mass:
The mass of the electron increases with speed and is governed by: m= m0 ˟ 1+ v ÷ c ˟ c.
Where:
* m= relativistic mass:
* m= rest mass:
m0
* v= velocity of the electron
* c= the speed of light
At normal speeds, the mass increase is so small that it is not significant. However, at speeds nearing the speed of light, the mass becomes considerably greater than rest mass.
Is the Relative Mass of an Electron 1/1840?
Yes, it is true that the relative mass of an electron is quoted as 1/1840 in comparison with a proton or neutron. This approximation stems from:
- The mass of a proton is approximately 1.672 × 10^-27 kilograms.
- The mass of an electron is approximately 9.109 × 10^-31 kilograms.
When you divide the mass of an electron by the mass of a proton, you get:
9.109×10−311.672×10−27≈11836
1.672×10
−27
9.109×10
−31
≈
1836
1
For educational purposes, like GCSEs, it is common practice to simplify calculations, which is why this is rounded to 1/1840.
Is the Relative Mass of an Electron 1/2000?
As stated previously, in some contexts the value of 1/2000 serves as an approximation for the mass of an electron. This is particularly prevalent in older texts. As simple as it is, this value is not as accurate as 1/1840.
This discrepancy arises because:
1. This figure is an oversimplification, as the mass ratio of an electron to a proton is more accurately 1/1836.
2. Rounding these calculations to 1/1840 provides more validity than rounding to 1/2000.
For most scientific calculations, the value 1/1840 is more useful. It’s true that 1/2000 is easier to calculate, but it’s less precise.
Why the Relative Mass of an Electron Matters
With regard to different fields in science, the relative mass of an electron clearly stands out as important in the following ways:
1. Atomic Structure:
The relative mass of electrons assists in understanding the structure of atoms. Electrons, which have a small mass, are able to rapidly revolve around the nucleus made up of protons and neutrons.
2. Chemical Bonding:
The bonds formed between atoms to create molecules are dictated by electrons’ actions. Because electrons have a small mass, they are able to undergo chemical reactions and compound formation.
3. Particle Physics:
In particle physics, electrons’ mass is important for analyzing the behavior of subatomic particles and the forces acting on them.
4. Technology:
So many devices today such as computers, smartphones, and even medical equipment rely on electricity and electronics. Hence, understanding electrons’ mass and behavior allow for the development of these technologies.
How Measuring Mass of an Electron is Done?
Calculating and measuring the mass of an electron is done through experimental work and theoretical calculations. One of the best known methods is Robert Millikan’s experiment in 1909 with oil drops. Here is how it goes:
1. Charged Oil Drops:
Microscopic oil droplets are charged and placed in an electric field.
2. Balancing Forces:
The charge of the oil drops can be determined by balancing the electric and gravitational forces acting on them.
3. Calculating Mass:
Using the delightful oil drop accommodating electron charge value junction, the mass of an electron can be calculated.
Comparing Electron Mass to Other Particles
Methods available today, for example, mass spectrometry, allow for much more precise measurement of the value of mass of an electron.
With regards to his or her mass, let me bring to your attention this little comparison with other subatomic particles:
1. Proton:
* Mass: ~ 1.672 × 10^-27 Kg
* Relative Mass: 1 amu
2. Neutron:
* Mass: ~ 1.675 × 10^-27 Kg
* Ame: 1 amu
3. Electron:
* Mass: ~ 9.109 × 10^-31 kg
* Relative Mass: ~ 1/1840 amu
This table clearly demonstrates how much lighter the electron is when compared to both the proton and neutron.
Common Misconceptions About Electron Mass
1. Electrons Having No Mass:
Electrons are significantly lighter in comparison to protons and neutrons, but they still have mass. The tiny mass of electrons is critical for their interactions in bound systems such as atoms, as well as in chemical processes.
2. Relative Measuring and Rest Mass is the Same:
An electron’s relative mass (1/1840) is the ratio between its mass and that of a proton or neutron, while rest mass is the mass of the electron when at rest.
3. Always Significant Relativistic Mass:
It’s only when an object is traveling close to the speed of light that relativistic mass becomes important. For most practical purposes, everyday motions render its rest mass adequate.
Conclusion
The electron’s relative mass is a basic notion that aids in grasping the structure of atoms, chemistry, and the behavior of these small, sub-atomic entities. From the perspective of GCSE science or more sophisticated realms of physics, it is vital to note that the rough estimate of electron’s relative mass is 1/1840 (or frequently simplified further to 1/2000).
From its contribution towards the composition of atoms to its effect on contemporary devices, an electron’s minute mass greatly transforms the perception of the universe. As its science advances, the measurement and understanding of the mass of an electron will always be one of the fundamentals of this ever-changing world of physics and chemistry.


Post Comment