Rest Mass of the Electron | A Fundamental Look at Its Absolute & Bare Mass
One typically finds an electron as one of the basic building blocks of matter. It is one of the most crucial particles that define the basic structure of an atom. Electrons have many unique properties but one of the essential ones is defined rest mass. How does an electron rest, or not is motion, changes its mass? What is the rest mass of an electron? In this piece, these questions will be answered.
What Is The Rest Mass Of A Free Electron?
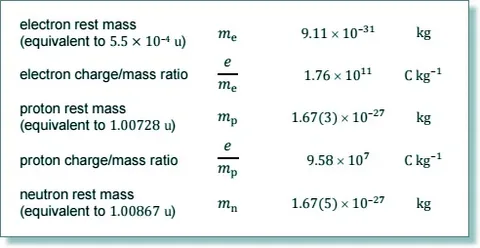
The rest mass of an electron is expressed in physics circles or in form of scientific notation as mₑ. This means the mass at the electron is stationary in relation to an observer. Recent measurements conducted through quantum electro dynamics techniques have rest mass of a free electron as depicted below:
9.109 × 10⁻³¹ kilograms (kg)
or equivalently,
0.511 MeV/c² (Mega electron volts per speed of light squared)
While this value is found to be exceptionally low when one considers the mass of neutrons and protons, the finding does make sense given the fact that electrons tend to be much lesser in atomic weight compared with the other subatomic particles, and thus, find themselves dwarfed in contrast to atomic nuclei. Atomic nature being the reason behind such a dramatically small value of rest mass tends to set forth the most acceptable explanation of this theory.
How Much Does an Electron Weigh?
Absolute mass is essentially rest mass when in motion, and in this case the electron’s mass is \(9.109 \times 10^{-31} kg\) since it would not change in value regardless of speed or changes in energy levels. To indicate a change in energy levels, consider a proton whose mass is \(1.673 \times 10^{-27} kg\) and is approximately 1836 times the weight of an electron. This ratio of mass is significant in the structure of an atom and in any resulting chemical reaction.
What is the Bare Mass of an Electron?
In quantum electrodynamics, the concept of bare weight comes to life as the unbounded weight of the electron and defines the weight that does not depend on the interaction of the particle with the vacuum nor electromagnetic field. In practice, a particle never works in isolation or the quantum vacuum which leads to what is called mass renormalization.
Increasingly sophisticated virtual particles such as photons surround electrons and alter their effective mass. The mass we measure in reality is dressed mass or physical mass, which accounts for these quantum effects. The bare mass of an electron, however, is devoid of such effects and is not directly measurable. This notion of bare mass is a paradigm from quantum field theory that lacks empirical validation.
Do Electrons Possess Rest Mass?
Electrons possess rest mass and, unlike photons, which do not have mass, electrons do carry non-zero rest mass. This is of utmost importance because if electrons did have no mass, atoms in their known forms would not exist, and the nature of matter would drastically change.
Having rest mass allows electrons to occupy atomic nuclei at quantized energy levels, leading to the creation of the periodic table and chemical bonding. Without these properties, the foundations of chemistry and physics would be rendered void.
How the Rest Mass of an Electron Is Measured?
To date, scientists have come up with a variety of ways to measure the rest mass of an electron with utmost precision. Some of the well-known techniques include:
Experiments using the charge-to-mass ratio – Using cathode rays and electromagnetic fields, it is possible to measure the ratio of the charge to mass of an electron. Mass can then be calculated with independent charge measurements.
Energy-Momentum Relations – Physicists measure the energy and momentum of electrons using particle accelerators and compute their rest mass using Einstein’s equation E = mc².
Penning Traps – These devices utilize electric and magnetic fields to confine electrons which permit measuring their mass accurately using their cyclotron frequency.
Why is the Electron’s Rest Mass Important?
Knowing the rest mass of an electron is essential for diverse fields of physics, such as:
Quantum Mechanics: The mass of the electron determines its wave-particle duality and role in quantum fields.
Electromagnetism: Moving electrons create electric and magnetic fields, which are essential for circuits as well as wireless transmission.
Astrophysics: The behavior of electrons in stellar settings influences neutron stars, black holes, and the supernova explosion.
Material Science: The physics of semiconductors, superconductors, and other cutting-edge materials are contingent on the mass of the electron.
Can the Electron’s Mass Change?
The mass of an electron is constant. However, within a relativistic framework, it can change relative to the motion of the electron.As it approaches the speed of light, its mass increases.
This effect is particularly important when it comes to particle accelerators, wherein electrons travel at approximately 99.99% the speed of light.
Conclusion
An electron’s rest mass is one of the constants of nature which describe the system of elementary particles and serves as a backbone upon which the structure of an atom, its quantum mechanics and electromagnetism are built. It doesn’t matter if we call it the absolute mass or think of the notion of bare mass; electrons have a presence, albeit scant and minuscule, which assists in balancing the universe. This value remains fixed, thus permitting the existence of matter in a way we understand it. While you ponder upon electrons, do remember that their minute rest mass value is instrumental in sustaining the universe.


Post Comment