Electron Cloud Model | Origins, Modern View, and Why It Still Matters
The atomic world is a domain of great fascination and complexity. Different scientists, over time, have constructed various models to represent the different constituents of an atom and its structure, each bringing us one step closer to reality. One of the most potent and enduring ideas is the electron cloud model, which influenced and revolutionized the way we think of atomic structure and its components.
In this blog, we will discuss what the electron cloud model was in 1926, what Schrodinger’s atomic theory proposed, and whether the model holds true today. Furthermore, we will discuss in simple terms the evolution of the electron cloud model and whether it is applied in modern science.
What was the electron cloud model in 1926?
The electron cloud model in 1926 was a major shift from previously known atomic models. It was created after identifying the flaws present in Niels Bohr’s planetary model of an atom, which depicted electrons as encircling the nucleus in distinct orbital paths.
In 1926 Erwin Schrödinger came up with the idea:
- Electrons don’t move in defined pathways.
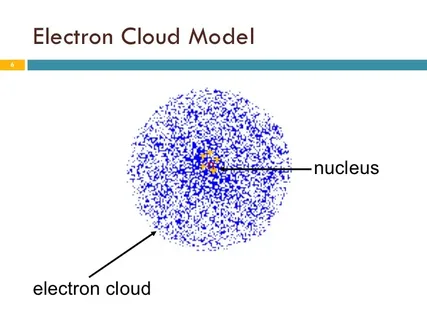
- Rather, they are located within a fog-like region surrounding the nucleus.
- Cross-determining their precise position is impossible; the only determinable feature is the likelihood of finding the electron within a particular region.
This ‘cloud’ model of electrons was more realistic in terms of atomic behaviour because it was developed on quantum mechanics, classical physics didn’t offer the same flexibility.
The model was advanced in a sense because it considered the erratic behaviour of electrons incorporating the level of uncertainty present, which in turn helped better understand the nature of chemical bonds and reactions.
What was Schrodinger’s atomic theory?
Schrodinger’s atomic theory was that electrons act more like waves than particles and stated that their positions were not fixed but fluid. Instead of distinct orbits, most probable zones of occupancy have to be identified for electrons.
He constructed the complicated mathematical equation known as the Schrödinger equation which:
- Describes how the quantum state of a system changes in time.
- Predicts the probable regions around the nucleus where electrons can be found.
These regions are termed orbitals, which is different from simple orbits. Each orbital has a different shape, like spherical or dumbbell-shaped, depending on the energy and type of electron.
In summary:
Schrödinger’s atomic theory substituted fixed paths of electrons with areas based on probabilities and laid down the structure for the current electron cloud model.
What is the basic essence of the electron cloud model?
If a straightforward answer is what you’re looking for, then here’s the essence of the electron cloud model—and I mean very simplified:
- Consider the nucleus of an atom as a tiny light bulb.
- Instead of tiny planets (electrons) orbiting around it like in the solar system, there’s a hazy, indistinct region enfolding the bulb.
- This must signify where the electrons are probable to exist, but at any specific moment, you cannot say exactly that.
There is a region of high probability and low probability where this “cloud” becomes dense and thin.
The model indicates that electrons are perpetually moving, and the most accurate prediction we can offer is where they will be at a given moment— not where they are exactly.
Would you consider the electron cloud model to be accurate?
As stated, the electron cloud model is one of the most accurate representations of atomic structure still accepted today, especially in basic and advanced chemistry.
This is what makes it credible:
- There are numerous experimental proofs that support it provided by quantum mechanics.
- Spectroscopy experiments corroborate the predictions of the cloud model.
- It does describe how electrons behave within atoms, especially in the highly intricate multi-electron systems.
Even so, we must stress the fact that while the electron cloud model may be effective, it is not factually the true picture of subatomic particles and is a simplification. There exist modern quantum field theories that are simpler and less approachable, but provide detailed explanations and models.
For most branches of chemistry and physics, the electron cloud model suffices in accuracy for plausible predictions and explanations of atomic actions.
What do we call the cloud of electrons today?
The cloud of an electron today has been built upon Schrodinger’s ideas over the years. The cloud concept today helps explain:
- The regions of space (orbitals) where electrons stands the greatest chance of being situated
- Different shapes of orbitals depending on the energy levels of the electrons (s, p, d, and f orbitals)
- A vacuum of certainty where an electron is likely to be found.
With the technological advancements of today with computers, there are now more accurate visualisations showing clouds complex patterns of atoms and molecules.
Other modern visualisations include:
- S orbitals have spherical clouds while
- P orbitals have dumbell shaped clouds.
- D orbitals are cloverleaf or complex shapes while
- F orbitals have even more complicated patterns.
These orbital shapes together help scientists and engineers at a molecular level to comprehend and demystify chemical bonding, material science, and even biological processes.
Do we still make use of the electron cloud model?
Definitely, we make use of the electron cloud model, without a question.
In fact it allows for:
- Theories concerning the chemical bonds like Hybridisation and the Molecular Orbital Theory
- Forecasting chemical reactions and the atomic molecular framework
- Aerospace material science, such as the invention of semiconductors and superconductors
- Technologies in medicine like the MRI machines which have quantum mechanics embedded in them.
All learners are taught the electron cloud model at the secondary and tertiary levels of Chemistry and it is taught to all students, while global scientific researchers apply much sophisticated versions of the model.
The remarkable attribute of the model is its simplicity. It is simple enough to learn and powerful enough to depict realistic situations in science.
Key features of the electron cloud model
| Feature | Description |
| Electrons move freely | No fixed paths, only probable locations |
| Probability-based | Higher density of cloud = higher probability of finding electrons |
| Multiple shapes | Orbitals (s, p, d, f) have different 3D shapes |
| Quantum mechanics | Built on Schrodinger’s wave equations |
| Real-world use | Still used in chemistry, physics, and engineering today |
Comparing The Bohr Model And The Electron Cloud Model
| Aspect | Bohr Model | Electron Cloud Model |
| Electron paths | Fixed circular orbits | Probabilistic regions (clouds) |
| Energy levels | Discrete rings around the nucleus | Energy levels with different orbital shapes |
| Accuracy | Good for hydrogen atoms only | Good for all atoms and molecules |
| View of electrons | Particles moving like planets | Particles acting like waves and particles |
The electron cloud model corrected the oversimplifications of Bohr’s model and opened the door to modern quantum chemistry.
Real world applications of the electron cloud model
The electron cloud model helps in many fields:
- Medicine: MRI Machines depend on a quantum understanding of electrons and nuclei.
- Technology: Microchips and semiconductors are designed using principles based on electron behavior.
- Energy: Solar panels and batteries are improved due to knowledge of electron arrangements.
- Pharmaceutics: Drug design is molecular modeling including concepts of electron clouds.
Without the electron cloud, modern science of today is altered in many ways.
Did you know facts of the electron cloud model
- Motion of the electron cloud is always in a certain period of speed and never completely still.
- Heisenberg’s uncertainty principle says that either position or momentum of an electron cannot be precisely known.
- Schrödinger’s theory brought forward the idea of electrons having dual nature of particle and wave which was proven by various experiments including double-slit experiment.
Importance of the Electron cloud model
The 1926 model of an atom proposed by Schrodinger regarded the atomic structure differently by introducing the probability model wherein electrons were envisioned as orbiting the nucleus like clouds, not in well defined paths. Even amatuer, like a 14 year old, his understanding would be simple, it would be drawing the electrons as orbiting the nucleus in woolly clouds of probability rather than in rather predictable orbits as satellites. The irrefutable beauty of this model lies that with the passing decades, instead of bos settling solving various puzzles leading to its establishing boundaries, time has edged it towards further leaps in proving it right.
Even at 100 years old, the region contour model still has its accuracy as its advantage. Outdated it may be, it’s still heavily relied on in schooling, science, and commercial industries. As science develops, the specifics of the electron cloud model is still widely used to explain the amazing phenomena of atoms.



Post Comment