How Electron Shielding Influences Electronegativity Across the Periodic Table
We will review the phenomena of Electrons Shielding, or Screening, along with the it’s definition in broader terms, along with its impact on ionic bonds, the relative distance between atoms, the increase of atomic number, a few factors it influences, and many more questions.
Aside from physics defining atoms as the simplest units, they form complex structures with a three-dimensional matrix of interacting protons, electrons, and neutrons, which isn’t a simple matter. The electronically constructed neutral atom is still warped by force fields, everything is warped by attractive and repulsive forces.
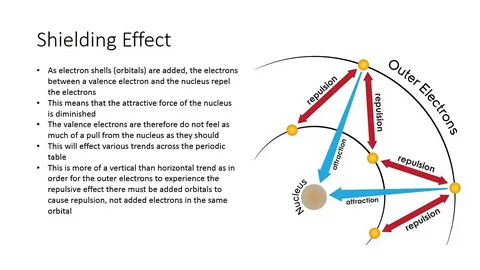
What is meant by shielding effect?
Every electron “inner” block is capable of raising energy of “outer” electrons beyond their relativistic inertia. Hence reducing the effective pull of the nucleus making the inner and outer shell gap positive, this also causes “electronic shielding”
In relation to valence electrons,
Definition:
Due to each subsequent inner electron layer, the weaker the attraction of full shell outer electrons towards the nucleus.
What is shielding in terms of electrons?
This occurs due to the following reasons:
Actions taken by the positively charged nucleus are offset by the outer electrons being repelled by the inner electrons because of their negative charge.
Outer electrons feel a weaker Z* because of added repulsion and less pulling of the nucleus.
Why does electron shielding increase down a group?
Because new subshells are added the further down a group one looks.
These inner electrons are more effective at shielding the outer electrons from the nucleus due to introducing new shells.
Example:
- One valence electron is blocked by two inner electrons for lithium.
- Ten inner electrons block a single valence electron for sodium.
- This means greater shielding is experienced by sodium over lithium.
- Consequently, down a group outer electrons are more loosely bound.
What increases electron shielding?
Important factors that can impact electron shielding include:
- With additional shells, there comes lower energy shells which add another layer of inner electrons.
- Down a group with an atomic number increase, electrons are added to new shells with protons.
- Noble gases are known for their full inner shells so they can particularly be shielding.
Consequently, elements possessing additional shells and more core electrons have greater shielding.
What is the anomalous trend for electron affinity?
Electron affinity is the energy change that occurs when an atom gains an electron.
The trend across a period:
- Increases, or becomes less negative, from left to right, as atoms desire to gain electrons and fill up their outer shells.
The trend down a group:
Generally decreases down a group for the reason:
- Increased shielding decreases the nucleus’ pull, making it more difficult to attract additional electrons.
- Therefore, electron shielding decreases electron affinity down a group.
In what way does shielding effect electronegativity?
Electronegativity is defined as the tendency of an atom to attract shared electrons in a covalent bond.
When shielding increases:
- The outer electrons are held less tightly, meaning the atom becomes less electronegative.
- Consequently, electron shielding is what causes electronegativity to decrease down a group.
Inversely, left to right across a period, shielding does not change much, but the number of protons increases, thus increasing electronegativity.
What does shielding most greatly impact?
Shielding most significantly impacts:
- Atomic radius: Greater shielding results in larger atoms (outer electrons are held loosely) and greater atomic radius.
- Ionisation energy: Greater shielding reduces ionisation energy, or the energy required to remove electrons.
- Electronegativity: Greater shielding results in reduced electronegativity.
- Electron affinity: Greater shielding results in reduced electron affinity.
In this regard, shielding is very important in determining atomic size and chemical activity.
What is meant by poor shielding effect in chemistry?
A poor shielding effect takes place when the inner electrons do not adequately block the nucleus’s pull of the outer electrons.
This is most notable with:
- f and d orbitals (located within the transition metals as well as the lanthanides/actinides).
- These orbitals are more diffuse than s and p orbitals and therefore less effective at shielding.
Consequently
- Outer electrons experience a more powerful nuclear force than what is presumed.
- Ionization energies can be elevated despite increased atomic sizes.
What causes the effective nuclear charge Z star (Z*) to increase down a column?
Z* (Effective nuclear charge) is the net positive charge experienced by the electron.
While actual nuclear charge is known to increase down a group (more protons), the shielding effect also increases greatly.
Because new shells are added and shielding becomes more effective, the increase in Z* is commonly small when compared to across a period.
In summary:
- Z* increases by a small amount down a group.
- Z* increases significantly when moving across a period.
Which protons are most shielded?
The protons’ effect on the outermost electrons is shielded the most when:
- There are a lot of inner electron shells.
- The atom is large, for example, cesium or barium.
- There are several core electrons between the nucleus and the valence electrons.
Thus, the protons in heavy elements, found lower down the periodic table, have their outer electrons most shielded from their influence.
Which element has the maximum shielding effect?
Francium (Fr), located at the bottom of Group 1, is the element with the maximum shielding effect.
- Francium has several inner electron shells.
- Its valence electron is at a huge distance from the nucleus.
Other highly shielded elements include:
- Cesium (Cs)
- Radium (Ra)
These elements possess large atomic sizes with very low ionization energies.
Real life examples of electron shielding.
- Alkali Metals
- Lithium vs. Cesium
Despite being in the same group, Cesium is much larger because of stronger shielding.
Periodic Trends:
- Fluorine is small and highly electronegative due to low shielding.
- Francium is large and weakly electronegative because of strong shielding.
Material Science:
- The shielding effect governs how easily an atom’s outer electrons can be removed, which in turn affects the conductivity and reactivity of metals.
Electron shielding summation – Takeaways
| Question | Quick Answer |
| What is electron shielding? | Inner electrons reduce nuclear pull on outer electrons |
| Why does electron shielding increase down a group? | More electron shells are added |
| How does shielding effect electronegativity? | More shielding → lower electronegativity |
| What trend exists for electron affinity? | Decreases down a group due to increased shielding |
| What causes poor shielding? | Diffuse d and f orbitals |
| Which element has the greatest shielding? | Francium (Fr) |
Why does electron shielding shape chemistry?
The shielding effect is a crucial concept that explains why some complex phenomena are periodic. Such as atomic size and chemical reactivity. It is observed that as you move down a divisional group, an increase in shielding causes atomic size to increase and results in weaker attractions to electrons.
Explaining what electrons shielding means, why the shielding effect becomes more profound down a column, and its relation with covalence, electronegativity, plus ionization energy enables one to grasp the periodic table, chemical reactions, and practically material properties and molecular structures.




Post Comment