Sulfur Electron Configuration – Mechanism and Significance
In the study of elements, the atom is one of the most critical parts as it defines the nature of element and bonding, alongside nuclear interactions. For sulfur, its atom’s most defining feature is its electron configuration, meaning arrangement of electrons around the nucleus.
In this guide, we will learn about sulfur and its significance as one of the nonmetals we frequently come across in daily life. Particularly, we will break down the task of writing out its configuration and comparing it to other elements with different configurations. To start, we will cover the building blocks.
How do you write the electron configuration for sulfur?
To arrive at a configuration for a sulfur atom, consider the following instructions:
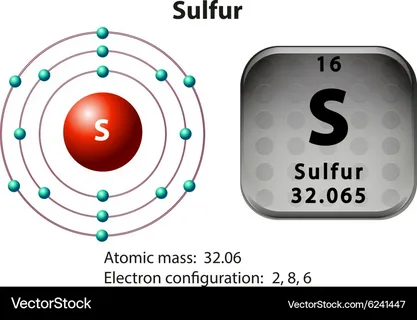
Step 1: Look up the atomic number for sulfur
Step 2: Note down the atomic number of the element which equals 16, it further signifies the number of electrons a neutral sulfur atom consists of. Hence, a sulfur atom will have 16 electrons.
Using the orbital filling method as per the energy levels and order of increasing principal using Aufbau principle, the orbitals must be filled based on the order of energy levels.
Sulfur’s electron configuration is;
1s² 2s² 2p⁶ 3s² 3p⁴
Stepwise breakdown:
- 1s² → 2 electrons
- 2s² → 2 electrons
- 2p⁶ → 6 electrons
- 3s² → 2 electrons
- 3p⁴ → 4 electrons
= Total: 16 electrons
Total of 6 electrons are contributed by 3s and 3p orbitals, thus making 6 valence electrons. showing that sulfur will typically form 2 bonds in inorder to attain 2 electrons needed to complete its outer.
What element does 1s² 2s² 2p⁶ 3s² 3p⁴ represent?
Would form an important highlight looking to identify elements with their configurations
This configuration 1s² 2s² 2p⁶ 3s² 3p⁴ is 16 electrons confirmed with atomic number of sulfur.
So, this element is represented by such a configuration.
Details under 1s² 2s² 2p⁶ 3s² 3p³ together are:
3s² → 2
3p³ → 3
= Total: 15 electrons
Making Phosphorus (p), the answer to 15. That’s the configuration, 1s² 2s² 2p 6 3s 2 3p 3
It sits very close on the periodic table with only one less electron, which is why sulfur and phosphorus are on the same period but in different groups.
Identifying the placement of sulfur on the table
Sulfur can be found in:
- 16th group (Chalcogens)
- 3rd Period
- S is the symbol
- Atomic number 16 indicates
Being a nonmetal, sulfur is important for its inorganic and organic chemistry and compounds. It is found in many compounds like;
- Sulfuric acid(H₂SO₄)
- Sulfurous acid H₂SO₃
- Sulfur dioxide (SO₂)
- Hydrogen sulfide (H₂S)
This is due to sulfur’s property of gaining electrons to share them in order to achieve a full outer shell.
What is the use of electron configuration for sulfur?
Some characteristics that can be predicted with knowing sulfur’s electron configuration are;
- Counting the number of bonds
- In which oxidation state it will be
- Will it lose or gain electrons?
- Chemical behaviors vise reactions
- How many bonds it can form
With the 6 valence electrons sulfur possesses, disulfide is often formed an ionic compound which is -2 anions (S²⁻). It is also possible to form double and single bonds in covalent compounds.
Which element possesses the electron configuration of 1s² 2s² 2p² 4s¹ 3d¹ 4p¹ 5s² 4d¹⁰?
Let’s take a look at this long configuration step by step.
= Total: 46 electrons
- 1s² → 2
- 2s² → 2
- 2p⁶ → 6
- 3s² → 2
- 3p⁶ → 6
- 4s¹ → 1
- 3d¹⁰ → 10
- 4p⁶ → 6
- 5s¹ → 1
- 4d¹⁰ → 10
46 is the total for configuration, which corresponds to palladium (Pd), but with an extra electron (5s¹) suggesting otherwise.
The most likely candidate is silver (Ag), which is 47.
Thus, the configuration is silver (Ag) while the rest filled in are from the previous statement: configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰ 4p 5s 4¹d¹⁰.
Remembering rules on electron configurations
Three main rules underline an electron’s configuration:
1. Aufbau Principle
The reason why orbitals fill in a sequential 1s, 2s, is it’s from lowest to highest energy.
2. Pauli Exclusion Principle
Two electrons can occupy an orbital under the condition that they must be spitting in opposite directions.
3. The energies of the electrons in these orbitals should be equivalent before pairing up.
A stable and predictable arrangement is achieved using these rules.
Noble gas shorthand for sulfur
You can express sulfur’s configuration using shorthand by referencing the closest noble gas preceding sulfur, which is Neon:
Neon (Ne) = 1s² 2s² 2p⁶.
Thus, we can wrap up the shorthand for sulfur’s electron configuration as:
[Ne] 3s² 3p⁴.
This notation becomes particularly favorable in chemistry due to the need to save time and streamline repetitive work.
How sulfur’s configuration affects bonding?
Sulfur is capable of forming:
- Ionic bonds (for example, in sodium sulfide, Na₂S).
- Covalent bonds (as in SO₂ and H₂S).
- Various oxidation states: -2, +4, +6.
This demonstrates how it is able to have versatile oxidation states. Its chemistry is very flexible due to how much it can share and accept electrons around the 3p level, especially being so close with its 3p orbital being at 3p4.
Sulfur in biological systems
Additionally, sulfur is vital to biology because:
- It can participate in the form of amino acids, including cysteine and methionine.
- It contributes to the structure of proteins by forming disulfide bonds.
- It plays a role in the activity of certain enzymes and respiration of the cell.
Everything mentioned relates to the atom’s sulfur’s electron configuration which determines its reactivity with other elements within live organisms.
How does sulfur relate to the proximate elements?
Oxygen (O)
Electron configuration: 1s² 2s² 2p⁴
Valence electrons: 6
Just like sulfur, oxygen needs 2 electrons as well to fill its outer shell which explains similar chemical behavior.
Phosphorus (P)
Electron configuration: 1s² 2s² 2p⁶ 3s² 3p³
Valence electrons: 5
Phosphorus is right next to sulfur in the periodic table and shares some chemical similarities, but the number of valence electrons markedly impacts how it bonds.
Uses of sulfur based on its chemical structure
Because of its explosive tendency and electron configuration, sulfur is employed in:
- Fertilizers – Sulphates enhance soil quality.
- Medicine – In antibacterials and creams.
- Battery Technology – In lithium sulfur batteries.
- Rubber Industry – In processes of vulcanization.
- Explosives – Like black powder.
All these possible applications stem from sulfur’s capacity to form stable compounds which is a consequence of his electron structure.
Learning sulfur was possible because of electrons
He may look like any other element, but with understanding his configuration of electrons, a multitude of pieces unfold – how it bonds, how it reacts, how it sustains life and industries.
As a brief summary:
- Sulfur’s electron configuration can be defined as 1s² 2s² 2p⁶ 3s² 3p⁴.
- It has 6 valence electrons which makes it versatile and reactive.
- Understanding sulfur’s electron configuration enhances your knowledge about its position in the periodic table and in the chemistry world.
And if you ever come across 1s² 2s² 2p⁶ 3s² 3p⁴ again, you’ll instantly recognize that it references sulfur.



Post Comment